Tepezza, a medication designed to combat Thyroid Eye Disease (TED), has sparked both hope and controversy within the medical community. TED is a challenging autoimmune disorder, marked by painful symptoms like swollen eyelids.
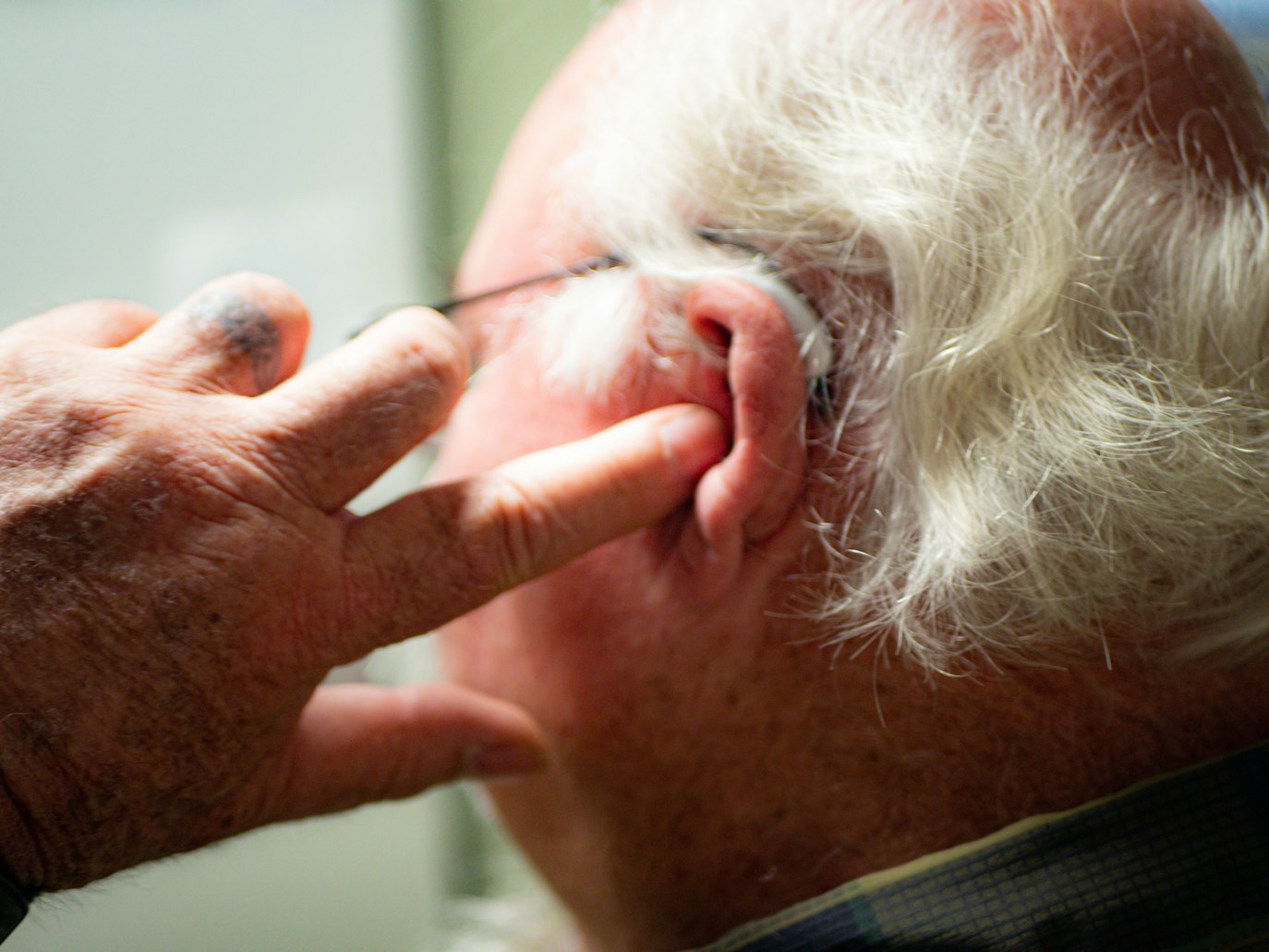
Tepezza, approved by the FDA in 2020, promises to tackle the underlying causes of TED, offering relief to those who endure these debilitating symptoms. However, recent scientific research claims that continuous Tepezza treatment has a high likelihood of causing permanent hearing loss.
In response, plaintiffs have filed lawsuits alleging that Tepezza, manufactured by Horizon Therapeutics, may be linked to permanent hearing loss. These lawsuits have triggered a legal battle that raises crucial questions about the medication’s safety and the transparency of information provided to patients.
In this article, we will delve into the world of Tepezza from a medical science perspective. We will explore the scientific foundation underlying the medication and examine the allegations of hearing loss.
Table of Contents
Understanding TED and Tepezza
Thyroid Eye Disease (TED), also known as Graves’ orbitopathy, is an autoimmune condition closely associated with thyroid dysfunction. TED causes immune system attacks on the soft tissues surrounding the eye socket, leading to a range of distressing symptoms. This includes pain, inflammation, bulging eyes, and double vision.
In 2020, the U.S. Food and Drug Administration (FDA) approved a groundbreaking treatment for this condition called Tepezza. What sets Tepezza apart from other treatments is its ability to target the underlying cause of TED, rather than merely alleviating symptoms. By blocking specific molecular switches responsible for the inflammation, Tepezza offers relief to those suffering from this autoimmune disorder.
Tepezza’s Mechanism and Administration
According to Healthline, Tepezza is administered through a slow intravenous infusion that typically lasts for 90 minutes. These treatments are scheduled at three-week intervals and can extend to a total of eight infusions. Clinical studies have demonstrated that symptoms continue to improve throughout the course of treatment.
By targeting the molecular switches that trigger inflammation and swelling behind the eye, Tepezza effectively addresses the root cause of TED. Double vision, ocular discomfort, and swelling are significantly reduced as a result. However, with this same Tepezza therapy, some individuals have reportedly developed permanent hearing loss.
Medical Science Behind Tepezza and Hearing Loss
Studies have explored the link between Tepezza and hearing impairment, with findings indicating that this connection is not to be taken lightly.
While clinical trials initially reported hearing issues in approximately 10% of patients, a 2021 study suggested a much higher risk. According to an article by the Endocrine Society, up to 65% of patients are experiencing hearing problems.
Horizon Therapeutics changed the label for Tepezza in July 2023 to add a warning regarding severe hearing damage. This suggests that the drug may indeed have an impact on hearing, making these lawsuits a significant concern.
The Emergence of Tepezza Lawsuits
Recent legal actions have raised concerns regarding Tepezza’s side effects. A significant number of plaintiffs have filed a Tepezza lawsuit against the drug’s manufacturer, Horizon Therapeutics.
The lawsuits filed by plaintiffs are alleging a connection between Tepezza treatment and hearing loss. The primary accusation in these lawsuits is that the manufacturer failed to warn the consumers about the risks associated with the drug.
The Current State of Tepezza Litigation
According to TorHoerman Law, the legal landscape surrounding Tepezza lawsuits is still evolving. Multiple cases have been consolidated into multidistrict litigation (MDL) in the Northern District of Illinois. According to the latest update from Drugwatch, 54 lawsuits are pending in the Tepezza MDL as of October 2023.
Plaintiffs continue to stand by their allegations of failure to warn on the part of Tepezza’s manufacturer. These legal actions serve to address the medical and financial consequences experienced by affected patients while raising awareness about the risks associated with Tepezza.
The Importance of Post-Market Research
The emergence of Tepezza hearing loss lawsuits underscores the significance of rigorous post-market research and monitoring for pharmaceutical products.
Such research is essential to ensuring patient safety by identifying and addressing potential side effects that may not have been apparent during clinical trials. Ongoing studies and post-approval surveillance are vital components of drug development and can contribute to a better understanding of a medication’s risk profile.
Balancing Benefits and Risks
Tepezza remains a vital treatment option for those suffering from TED, effectively addressing the root cause of the condition and providing substantial symptom relief. However, recent legal actions have highlighted the need to balance the potential benefits of the drug with its associated risks.
Patients considering Tepezza treatment should consult their healthcare providers, ensuring they are fully informed about potential side effects. Patients should opt for the Tepezza treatment only after making an informed decision that considers the benefits and risks of the medication.
Final Word
The Tepezza hearing loss lawsuits shed light on the delicate balance between medical advancement and patient safety. Tepezza’s promise to address Thyroid Eye Disease’s underlying causes has been overshadowed by the allegations of hearing loss.
This has triggered a legal battle that emphasizes the vital role of post-market research in monitoring drug safety. When choosing a course of therapy, patients need to carefully consider the risks and advantages.
These cases serve as a reminder of how important it is for pharmaceutical firms, healthcare practitioners, and consumers to communicate openly and honestly. It guarantees the welfare of those seeking treatment for incapacitating disorders and contributes to the preservation of faith in medical science.